
Principle
Zirconia (ZrO2) is a ceramic, doped with acertain percentage of low-valent metal oxides as stabilizers, such as calcium oxide (CaO), magnesium oxide (MgO), and yttrium oxide (Y2O3), it has high-temperature conductivity, a conductor with ionic conductive properties, and becomes a zirconia solid electrolyte. At a certain temperature, when the oxygen content in the gas on both sides of the zirconia tube is different, a typical oxygen concentration difference cell is formed.
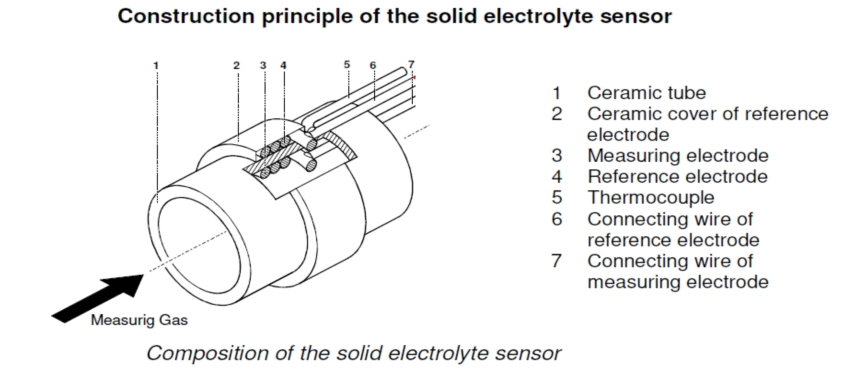
The zirconia tube as a whole is tubular, separated by zirconia material, and a porous layer of metal is sintered on each side of the zirconia as an electrode (usually Pt is used as the electrode material). At a certain temperature (600℃~1400℃), oxygen molecules on the side with higher oxygen content are adsorbed on the electrode, making the electrode on that side positively charged and the positive or anode of the oxygen concentration difference battery. Under the catalytic action of platinum, a reduction reaction occurs and electrons are obtained to form oxygen ions. The oxygen ions migrate through the holes in the zirconia crystal to the other side where the oxygen content is lower, making that electrode negatively charged and the negative or cathode of the oxygen concentration cell. At the platinum electrode electrons are lost and oxygen molecules are formed. This creates a certain potential at the two electrodes due to the buildup of positive and negative charges, which is related to the difference in oxygen content concentration in the two measured gases of zirconia, in accordance with Nernst equation:
E=(RT/4F)*Ln(P0/P)
Where
E------- oxygen concentration difference potential (mV)
R------- gas constant 8.3145 J/mol-K
T------- zirconia probe operating temperature (K, absolute temperature) = 273.15 + t (°C)
F------- Faraday constant, 96485.3365 (C/mol)
P0------- partial pressure of oxygen in the reference gas
P ------- Oxygen partial pressure in the sample gas
The oxygen partial pressure (P) in the gas to be measured can be calculated by measuring the concentration cell potential E and the absolute temperature of the zirconia probe, and thus the oxygen concentration in the gas to be measured.
The zirconia method has high sensitivity, fast response time, wide linear range, good reproducibility and stability. The internal structure is simple and almost independent of external environmental conditions such as temperature, vibration, etc., and requires little maintenance. However, the zirconia method is not suitable for measuring oxygen concentration in reducing gases or gas samples with high reducing gas content because the oxygen concentration is affected by the reducing gas in the gas to be measured, resulting in low measurement results. The sensor life is typically 5 years or more and is typically used for oxygen content measurements at ppm, air concentration (20.64%).
Our sensors have been delivered to more than 2,000 customers in industrial facilities and research institutions in 73 countries so far. And we can offer the catalytically inactive potentiometric oxygen measuring cell, which has a worldwide unique position, combines the advantages of classic high-temperature solid electrolyte sensors with those of cold electrochemical oxygen sensors (Clark cells).
Highlights:
• No drift, maintenance-free, no calibration required*
• Range: 0 ~ 10ppm/100ppm/1000ppm/30%(up to 100 vol% on request)
• Quick response: T90< 0.5s
• High accuracy and repeatability: Accuracy <3%FS, Repeatability <±0.1%FS
• Sample gas temperature: <300°C(Optional,700~1400°C)
• Working temperature: 700~800°C
• Gas pressure: <2bar(Available for vacuum)
• Flow: 5~10l/h, Max.10m/s
• Warm up time: 5minute
• Rugged and durable design
• Long-life zirconia sensor
*For vacuum application, need calibration
Main applications:
1. Inert gas measurement of trace oxygen content, electronic semiconductor industry, air separation, steel metallurgy, chemical fertilizer, chemical industry, welding protection, etc.
2. Flue gas measurement of percent oxygen content, power plants, petrochemical refining, chemical industry, iron and steel metallurgy, cement and building materials, boilers, etc.
Applications Industry:
• ASU(Air separation unit)
• Chemical, Pharmaceutical Industry
• Petroleum and Petrochemical Industry
• Metallurgical Industry
• Glass manufacturing
• Semiconductor Industry
• Food and beverage Industry
• Flare monitoring
• Nuclear,heat treatment, welding protection
• Environmental area monitoring
• Anesthesia, breathing and prenatal care
